Biology
Enzymes are proteins that vary widely in their particular structures, functions, and specificity; however, their general themes remain constant. More importantly, most of the biological reactions that occur, are governed by enzymes, making this an important topic to discuss.
STRUCTURE:
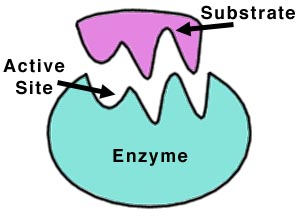
The structure of enzymes normally is made up of individual proteins (strands of polypeptides -- which are themselves strands of amino acids that have been strung together), to form what is known as a globular protein. Each enzyme also has areas on it that look like a bite has been taken out of them, known as the active site. This is where the reactant binds to the enzyme, noncovalently. The bonding between the enzyme and the reactant, will be discussed later in the 'Specificity' section. It is important to realize that although each enzyme has an active site for which to bind the reactant, each reaction/binding requires a different shaped active site depending on that exact type of reaction.
FUNCTION:
The main function of any enzyme is to lower the energy of activation for a particular biological reaction, thus increasing the rate of that reaction. The amount of increase in the rate of the reaction can be up to thousands of trillions faster! The amazing part about enzymes is that they are able to help out reactions in such a huge fashion, yet, they are not consumed in the reaction and can immediately be used for another one. This trend will continue until a point called saturation kinetics is reached (will be discussed in a later blog), but briefly, it is a point where substrates are so numerous, that they must wait in line for an available enzyme to bind.
CLASSIFICATION:
When classifying an enzyme, scientists use a nomenclature that simply uses a pre-fix that explains what the enzyme does. This can be a common word or a stem that relates to its function. At the end of the stem, the ending -ase is used to mean that it is an enzyme. For example, the word ligate means to bring things together. So when trying to name an enzyme that would bring perhaps, two proteins together (as is the case in DNA replication), scientists have named it 'ligase'. Here we will divide all enzymes into a total of 8 categories and along with their function.
1. oxidoreductase (oxidizes one molecule, while reducing another)
2. transferase (transfers a molecule)
3. hydrolase (breaks a hydrogen bond -- enzyme for word 'hydrolysis')
4. lyase (breaks up a molecule)
5. isomerase (changes structural arrangement of isomers)
6. ligase (brings molecules together)
7. kinase (adds a phosphate in process known as phosphorylation)
8. phosphotase (removes the aforementioned phosphate)
SPECIFICITY:
The bond between the active site on the enzyme and the substrate (on reactant) are highly specific. A good way to explain this is to look at the 'Lock and Key theory'. Basically, only a particular enzyme will bind to a particular substrate, allowing the enzymes to be more efficient (like a lock and key). Another theory is called the 'Induced Fit model', which says that the shape of both the enzyme and the substrate change upon binding, maintaining their specificity. Once the active site of the enzyme and the substrate come together, it is known as the enzyme-substrate complex.The take home message however, is that enzymes are very specific to what they're supposed to bind to, and unless there's a mutation in their genes during production, they don't mess up and stay highly consistent.
For the definitions to the bold-faced terms, check out the glossary. Also, soon to come on the subject, saturation kinetics, enzyme inhibition, and enzyme regulation. And don't forget, if you have any questions, please don't hesitate to email me at [email protected] and I will do my best to assist you.
- Enzyme Inhibition
In competitive inhibition, inhibitor has a shape, charge, size and structure similar to that of the substrate. It therefore competes with the substrate for the active sites to form the enzyme-inhibitor complex (E-I complex) and reduces the number of active...
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
- Enzymes
ENZYMES Enzymes are biological catalysts which influence biochemical reactions. All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes. Ribozymes: These are the Nucleic acids (RNA) that behave like...
Biology
Enzymes: Structure, Function, Classification, and Specificity
Enzymes are proteins that vary widely in their particular structures, functions, and specificity; however, their general themes remain constant. More importantly, most of the biological reactions that occur, are governed by enzymes, making this an important topic to discuss.
STRUCTURE:
The structure of enzymes normally is made up of individual proteins (strands of polypeptides -- which are themselves strands of amino acids that have been strung together), to form what is known as a globular protein. Each enzyme also has areas on it that look like a bite has been taken out of them, known as the active site. This is where the reactant binds to the enzyme, noncovalently. The bonding between the enzyme and the reactant, will be discussed later in the 'Specificity' section. It is important to realize that although each enzyme has an active site for which to bind the reactant, each reaction/binding requires a different shaped active site depending on that exact type of reaction.
 |
FUNCTION:
The main function of any enzyme is to lower the energy of activation for a particular biological reaction, thus increasing the rate of that reaction. The amount of increase in the rate of the reaction can be up to thousands of trillions faster! The amazing part about enzymes is that they are able to help out reactions in such a huge fashion, yet, they are not consumed in the reaction and can immediately be used for another one. This trend will continue until a point called saturation kinetics is reached (will be discussed in a later blog), but briefly, it is a point where substrates are so numerous, that they must wait in line for an available enzyme to bind.
CLASSIFICATION:
When classifying an enzyme, scientists use a nomenclature that simply uses a pre-fix that explains what the enzyme does. This can be a common word or a stem that relates to its function. At the end of the stem, the ending -ase is used to mean that it is an enzyme. For example, the word ligate means to bring things together. So when trying to name an enzyme that would bring perhaps, two proteins together (as is the case in DNA replication), scientists have named it 'ligase'. Here we will divide all enzymes into a total of 8 categories and along with their function.
1. oxidoreductase (oxidizes one molecule, while reducing another)
2. transferase (transfers a molecule)
3. hydrolase (breaks a hydrogen bond -- enzyme for word 'hydrolysis')
4. lyase (breaks up a molecule)
5. isomerase (changes structural arrangement of isomers)
6. ligase (brings molecules together)
7. kinase (adds a phosphate in process known as phosphorylation)
8. phosphotase (removes the aforementioned phosphate)
SPECIFICITY:
The bond between the active site on the enzyme and the substrate (on reactant) are highly specific. A good way to explain this is to look at the 'Lock and Key theory'. Basically, only a particular enzyme will bind to a particular substrate, allowing the enzymes to be more efficient (like a lock and key). Another theory is called the 'Induced Fit model', which says that the shape of both the enzyme and the substrate change upon binding, maintaining their specificity. Once the active site of the enzyme and the substrate come together, it is known as the enzyme-substrate complex.The take home message however, is that enzymes are very specific to what they're supposed to bind to, and unless there's a mutation in their genes during production, they don't mess up and stay highly consistent.
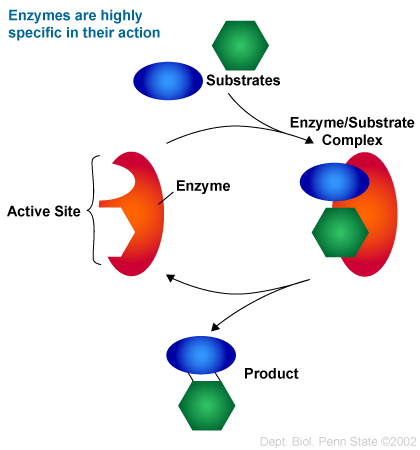 |
| Enzyme Specificity Depiction |
For the definitions to the bold-faced terms, check out the glossary. Also, soon to come on the subject, saturation kinetics, enzyme inhibition, and enzyme regulation. And don't forget, if you have any questions, please don't hesitate to email me at [email protected] and I will do my best to assist you.
- Enzyme Inhibition
In competitive inhibition, inhibitor has a shape, charge, size and structure similar to that of the substrate. It therefore competes with the substrate for the active sites to form the enzyme-inhibitor complex (E-I complex) and reduces the number of active...
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
- Enzymes
ENZYMES Enzymes are biological catalysts which influence biochemical reactions. All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes. Ribozymes: These are the Nucleic acids (RNA) that behave like...
