Biology
In the absence of enzyme, only 200 molecules of H2CO3 are formed in an hour. In the presence of carbonic anhydrase about 600,000 molecules are formed per second.
The enzyme action on substrate is similar to a Lock-Key action. It involves the following steps:
 Activation energy is the minimum energy required to start a chemical reaction.
Activation energy is the minimum energy required to start a chemical reaction.
a) Temperature and pH
Enzymes show highest activity at optimum temperature & pH. Activity declines below and above optimum value. 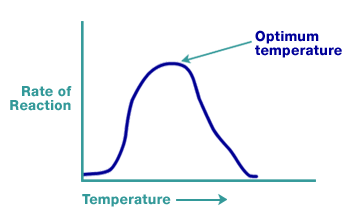
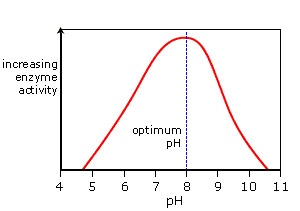
At low temperature, enzyme temporarily inactive.
At high temperature, enzymes destroy because proteins are denatured by heat.
b) Concentration of the substrate:

Co-factors are 3 types:
A) Prosthetic group: These are organic co-factor that are tightly bound to apoenzyme.
E.g. In peroxidase and catalase, haem is prosthetic group.
B) Co-enzymes: These are also organic compounds which are loosely bound to apoenzyme. The essential component of many co-enzymes is vitamins. E.g. NAD and NADP contain niacin.
C) Metal ions: They form co-ordination bonds with side chains at active site and the same time form one or more co- ordination bonds with the substrate.
E.g. Zn is a co-factor for Carboxypeptidase.
- Q: Explain The Effects Of Too High A Temperature On An Enzyme
If the temperature is too high, an increase in kinetic energy of the molecules will cause excessive vibrations of the molecules. This will disrupt the 3 dimensional structure of the enzyme, causing it to become a random coil. The enzyme is therefore denatured....
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #18. Enzymes - Active Site, Activation Energy, Enzyme Specificity
Enzymes are globular proteins that serve as biological catalysts. They speed up or slow down metabolic reaction, but remain unchanged. They may facilitate the breaking of an existing bond or the formation of a new bond....
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
Biology
Enzymes
ENZYMES

Enzymes are biological catalysts which influence biochemical reactions.
All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes.
All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes.
Ribozymes: These are the Nucleic acids (RNA) that behave like enzymes.
The substance on which the enzyme acts is known as substrate.
Enzymes are specific. i.e, Each enzyme can only acts on a specific substrate.
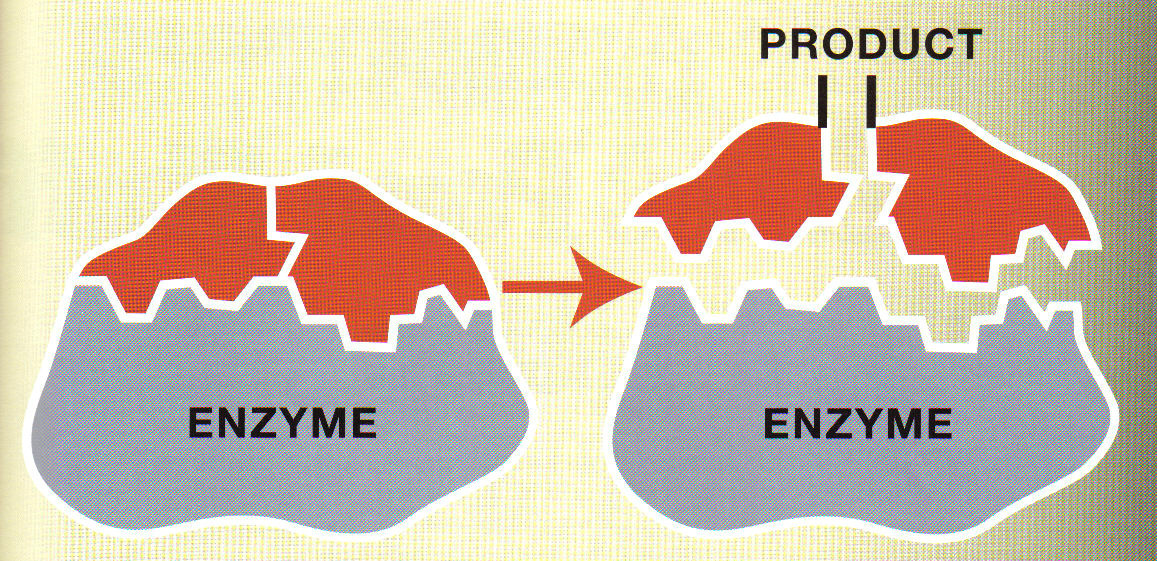
The tertiary structure of an enzyme has some crevices (pockets) called ?active site? into which the substrate fits.
Inorganic catalysts work at high temperature & pressure. But enzymes get damaged at high temperature. (> 400 C).
The substance on which the enzyme acts is known as substrate.
Enzymes are specific. i.e, Each enzyme can only acts on a specific substrate.
The tertiary structure of an enzyme has some crevices (pockets) called ?active site? into which the substrate fits.
Inorganic catalysts work at high temperature & pressure. But enzymes get damaged at high temperature. (> 400 C).
Thermophilic organisms have enzymes which are stable at high temperature (up to 80-900 C).
Carbonic anhydrase is the fastest enzyme. It accelerates the following reaction 10 million times.
Carbonic anhydrase is the fastest enzyme. It accelerates the following reaction 10 million times.
CO2 + H2O carbonic anhydrase H2CO3
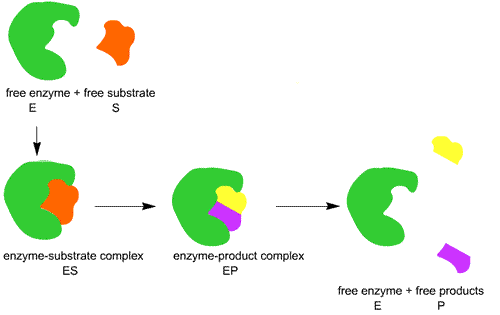
Nature of enzyme action (catalytic cycle)

E + S ? ES ? EP ? E + P
Step 1: The substrate binds to the active site of enzyme (E+S).
Step 2: This induces some changes in enzymes so that the substrate is tightly bound with active site of enzyme to form Enzyme-Substrate complex (ES).
Step 2: This induces some changes in enzymes so that the substrate is tightly bound with active site of enzyme to form Enzyme-Substrate complex (ES).
Step 3: The active site breaks chemical bonds of the substrate to form Enzyme-Product complex (EP).
Step 4: The enzyme releases the products and the free enzyme is ready to bind to other molecules of the substrate (E+P).
Step 4: The enzyme releases the products and the free enzyme is ready to bind to other molecules of the substrate (E+P).
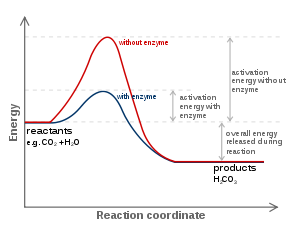
Role of Enzyme (Concept of activation energy)

In an exothermic or an endothermic (energy requiring) reaction, the ?S? has to go through a much higher energy state. It is called transition state energy. Therefore, activation energy is the difference between average energy of substrate and transition state energy.
If the product (P) is at a lower energy level than the substrate (S), the reaction is an exothermic reaction (spontaneous reaction). It requires no energy (by heating) in order to form the product. In a biochemical reaction, enzymes lower the activation energy. As a result, speed of the reaction increases.
Factors affecting enzyme activity
a) Temperature and pH
Enzymes show highest activity at optimum temperature & pH. Activity declines below and above optimum value.


At low temperature, enzyme temporarily inactive.
At high temperature, enzymes destroy because proteins are denatured by heat.
b) Concentration of the substrate:

With the increase in substrate concentration, the velocity of enzyme action rises at first and reaches a maximum velocity (Vmax). This is not exceeded by further rise in concentration because enzyme molecules are fewer than the substrate molecules. I.e. No free enzyme molecules to bind with additional substrate molecules.
c) Presence of Inhibitor:
The binding of specific chemicals (inhibitor) shuts off the enzyme activity. This is called inhibition.
c) Presence of Inhibitor:
The binding of specific chemicals (inhibitor) shuts off the enzyme activity. This is called inhibition.
If the inhibitor closely similar to the substrate it is called competitive inhibitor. It competes with the substrate for the binding site of the enzyme. As a result, the substrate cannot bind and the enzyme action declines.
E.g. Inhibition of succinic dehydrogenase by malonate which is similar to the substrate succinate.
Competitive inhibitors are used to control bacterial pathogens.
1. Oxido-reductases / Dehydrogenases: They catalyze oxido-reduction between two substrates.
2. Transferases: They catalyze a transfer of a group (other than hydrogen).
3. Hydrolases: They catalyze hydrolysis of ester, ether, peptide, glycosidic, C-C, C-halide or P-N bonds.
4. Lyases: They catalyze removal of groups by mechanisms other than hydrolysis leaving double bonds.
5. Isomerases: They catalyze inter-conversion of optical geometric or positional isomers.
6. Ligases: They catalyze the linking together of 2 compounds.
E.g. enzymes catalyzing joining of C-O, C-S, C-N, P-O etc.
Co-factors are non-protein constituents bound to the enzyme to make the enzyme active.
The protein portion of the enzyme is called Apo-enzyme.
E.g. Inhibition of succinic dehydrogenase by malonate which is similar to the substrate succinate.
Competitive inhibitors are used to control bacterial pathogens.
Classification and nomenclature of enzymes
1. Oxido-reductases / Dehydrogenases: They catalyze oxido-reduction between two substrates.
S reduced + S? oxidized ? S oxidized + S? reduced
2. Transferases: They catalyze a transfer of a group (other than hydrogen).
S-G + S? ? S?-G + S
3. Hydrolases: They catalyze hydrolysis of ester, ether, peptide, glycosidic, C-C, C-halide or P-N bonds.
4. Lyases: They catalyze removal of groups by mechanisms other than hydrolysis leaving double bonds.
X-C-C-Y ? X-Y + C=C
5. Isomerases: They catalyze inter-conversion of optical geometric or positional isomers.
6. Ligases: They catalyze the linking together of 2 compounds.
E.g. enzymes catalyzing joining of C-O, C-S, C-N, P-O etc.
Co-factors
Co-factors are non-protein constituents bound to the enzyme to make the enzyme active.
The protein portion of the enzyme is called Apo-enzyme.
Co-factor + Apoenzyme (inactive) = Holoenzyme (Active)
Co-factors are 3 types:
A) Prosthetic group: These are organic co-factor that are tightly bound to apoenzyme.
E.g. In peroxidase and catalase, haem is prosthetic group.
B) Co-enzymes: These are also organic compounds which are loosely bound to apoenzyme. The essential component of many co-enzymes is vitamins. E.g. NAD and NADP contain niacin.
C) Metal ions: They form co-ordination bonds with side chains at active site and the same time form one or more co- ordination bonds with the substrate.
E.g. Zn is a co-factor for Carboxypeptidase.
- Q: Explain The Effects Of Too High A Temperature On An Enzyme
If the temperature is too high, an increase in kinetic energy of the molecules will cause excessive vibrations of the molecules. This will disrupt the 3 dimensional structure of the enzyme, causing it to become a random coil. The enzyme is therefore denatured....
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #18. Enzymes - Active Site, Activation Energy, Enzyme Specificity
Enzymes are globular proteins that serve as biological catalysts. They speed up or slow down metabolic reaction, but remain unchanged. They may facilitate the breaking of an existing bond or the formation of a new bond....
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
