Biology
1. Storage Polysaccharides
Glycogen (in animals and fungi)
2. Structural polysaccharides
Plant cell walls contain the polysaccharide cellulose: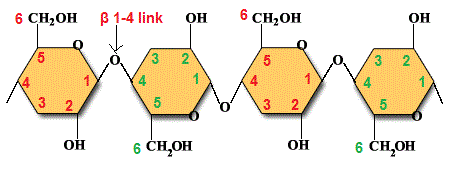
- Q: Compare And Contrast The Structure Of Collagen And Cellulose
The monomer of collagen is an amino acid whereas the monomer of cellulose is beta-glucose. The monomers in collagen are linked by peptide bonds whereas those of cellulose are beta (1,4)-glycosidic bonds. Collagen is an alpha helix polypeptide with a turn...
- #16 Summary Of Biological Molecules
From small to large 1. The larger biological molecules are made from smaller molecules. Polysaccharides are made from monosaccharides, proteins from amino acids, nucleic acids from nucleotides, lipids from fatty acids and glycerol. 2. Polysaccharides,...
- #8. Carbohydrates - Monosaccharides, Disaccharides
Carbohydrates: - Sugar polymers - Molecules contain C, H, O atoms - H atoms are twice as many as C or O atoms (C6H12O6) Monosaccharides The simplest carbohydrates They are sugar: C = 3 = triose C = 4 = tetrose C = 5...
- #7.2 Biological Molecules - Syllabus 2016
2.1 Testing for biological molecules 2.2 Carbohydrates and lipids 2.3 Proteins and water This section introduces carbohydrates, proteins and lipids: organic molecules that are important in cells. Nucleic...
- Comparison Between Starch, Glycogen And Cellulose
Comparison between Starch, Glycogen and Cellulose Characters Starch Glycogen Cellulose Monomer a-glucose a-glucose b-glucose Type of bond between monomers 1,4 glycosidic bond (amylose) + 1,4 and 1,6 glycosidic bond (amylopectin) 1,4 and...
Biology
#9. Carbohydrates - Polysaccharides

Molecules contain hundreds/thousands of monosaccharides linked into long chains.
Molecules are enormous --> the majority do not dissolve in water --> good for storing energy (starch and glycogen) or for forming strong structures (cellulose).
Molecules are enormous --> the majority do not dissolve in water --> good for storing energy (starch and glycogen) or for forming strong structures (cellulose).
1. Storage Polysaccharides
Glycogen (in animals and fungi)
- Made of ?-glucose molecules linked together by glycosidic bonds.
- Most of the bonds are ?1-4 links (C1 on one glucose + C4 on the next)
- There are some 1-6 links, which form branches in the chain.
- The bonds can be hydrolysed by carbohydrase enzymes to form monosaccharides, used in respiration.
- The branches increase the rate of hydrolysis.
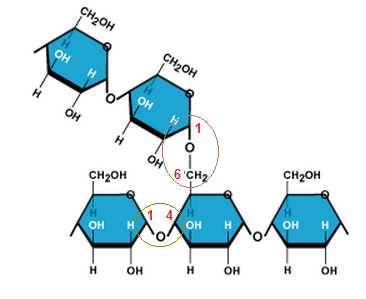 |
| 1-4 and 1-6 links in Glycogen. |
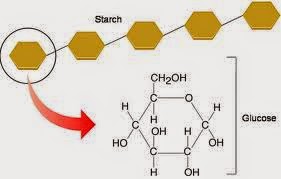
Starch (in plants)
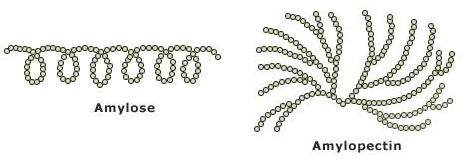
- A mixture of amylose and amylopectin. Both forms of starch are polymers of ?- Glucose. Natural starches contain 10-20% amylose and 80-90% amylopectin.

- Amylose molecule is a very long chain with 1-4 links. The chain coils up into a spiral, held in shape by H bonds between the glucose units.

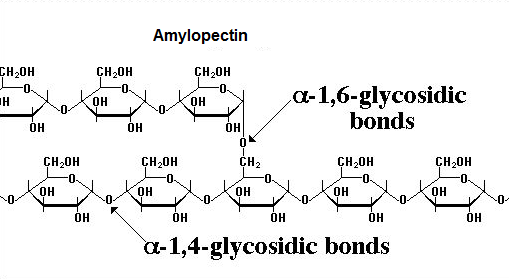
- Amylopectin differs from amylose in being highly branched. Short side chains of about 30 glucose units are attached with 1- 6 linkages approximately every 20-30 glucose units along the chain.

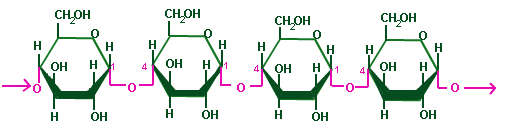
- Made of many ? glucose molecules, linked by ? 1-4 links.
- Adjacent glucose molecules in the chain are upside-down to one another.

- The chain is straight (not spiralling).
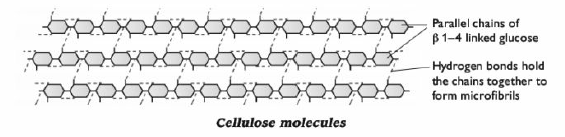
- H bonds between chains --> very strong microfibrils --> cell wall will not break easily if the plant cell absorbs water; difficult to digest (few organisms have enzyme that can break the ? 1-4 bonds).

Syllabus 2015 (c) describe the formation and breakage of a glycosidic bond with reference both to polysaccharides and to disaccharides including sucrose; (d) describe the molecular structure of polysaccharides including starch (amylose and amylopectin), glycogen and cellulose and relate these structures to their functions in living organisms; |
Syllabus 2016 c) describe the formation of a glycosidic bond by condensation, with reference both to polysaccharides and to disaccharides, including sucrose d) describe the breakage of glycosidic bonds in polysaccharides and disaccharides by hydrolysis, with reference to the non-reducing sugar test e) describe the molecular structure of polysaccharides including starch (amylose and amylopectin), glycogen and cellulose and relate these structures to their functions in living organisms |
- Q: Compare And Contrast The Structure Of Collagen And Cellulose
The monomer of collagen is an amino acid whereas the monomer of cellulose is beta-glucose. The monomers in collagen are linked by peptide bonds whereas those of cellulose are beta (1,4)-glycosidic bonds. Collagen is an alpha helix polypeptide with a turn...
- #16 Summary Of Biological Molecules
From small to large 1. The larger biological molecules are made from smaller molecules. Polysaccharides are made from monosaccharides, proteins from amino acids, nucleic acids from nucleotides, lipids from fatty acids and glycerol. 2. Polysaccharides,...
- #8. Carbohydrates - Monosaccharides, Disaccharides
Carbohydrates: - Sugar polymers - Molecules contain C, H, O atoms - H atoms are twice as many as C or O atoms (C6H12O6) Monosaccharides The simplest carbohydrates They are sugar: C = 3 = triose C = 4 = tetrose C = 5...
- #7.2 Biological Molecules - Syllabus 2016
2.1 Testing for biological molecules 2.2 Carbohydrates and lipids 2.3 Proteins and water This section introduces carbohydrates, proteins and lipids: organic molecules that are important in cells. Nucleic...
- Comparison Between Starch, Glycogen And Cellulose
Comparison between Starch, Glycogen and Cellulose Characters Starch Glycogen Cellulose Monomer a-glucose a-glucose b-glucose Type of bond between monomers 1,4 glycosidic bond (amylose) + 1,4 and 1,6 glycosidic bond (amylopectin) 1,4 and...
