Biology
Biology concepts ? dissolved gas, cavitation, arthritis, decompression sickness, ebulism, gas embolism
You approach the piano, interlace your fingers and bend your hands backwards, trying to crack your knuckles. Not satisfied, you pop each knuckle individually, followed by making small circles with each thumb and wrist.
Picture a syringe. When the plunger is pushed in there is low volume in the syringe. As the plunger is pulled out, the volume in the syringe increases. The air pressure inside the syringe is now lower than outside the syringe so air (or liquid) rushes in to equalize the pressure.
During the refractory period, the joints are a little larger and a little looser. The stretch and movement sensors in the tendons around the popped joint are stimulated, giving heightened sensory response in that joint, while the muscles around the joint under go a relaxation immediately after cracking. No wonder many people say they feel invigorated or relaxed after popping joints.
However, sometimes gas can be deadly. No, I?m not talking about THAT kind of gas! In your blood there is some dissolved oxygen traveling around unescorted. Ninety-nine percent is bound to hemoglobin, but still there is a little free oxygen as well. The oxygen is on its way to your cells to provide an electron acceptor during the production of ATP via oxidative phosphorylation.
Decompression sickness is a macro version of your knuckle joints, occurring all over your body. The bubbles have a tendency to form in, or move to, your joints, and this is painful. Remember that after you crack your knuckle, the volume goes back down and the gas is under greater pressure again and goes back into solution. No such luck in decompression sickness, the pressure remains lower than when you were diving and the bubbles take much, much longer to be resorbed by the body.
Every once in a while you hear about someone on a plane having a stroke or a heart attack. This might be someone returning from Hawaii or some other tropical paradise. If they had scuba dived in the morning (greatly increased pressure), and then to plane altitude (lower pressure) they could induce bubble formation even if they ascended correctly.
It gets worse for some guys. Certain drugs can affect the amount and solubility of gases in your blood, like nitric oxide (NO)-generating or -manipulating drugs. NO vasodilators increase the nitrogen gas in your blood. Name a nitric oxide-based vasodilator -?- yep, Viagra. A 2013 study has shown that pretreatment of rats with Viagra promotes decompression sickness when the pressure on their bodies is increased and then rapidly returned to normal. The question is, was it louder when the rats cracked their knuckles?
Next week, another question in biology - can bacteria change the earth - the whole earth?
Blatteau, J., Brubakk, A., Gempp, E., Castagna, O., Risso, J., & Vallée, N. (2013). Sidenafil Pre-Treatment Promotes Decompression Sickness in Rats PLoS ONE, 8 (4) DOI: 10.1371/journal.pone.0060639
deWeber, K., Olszewski, M., & Ortolano, R. (2011). Knuckle Cracking and Hand Osteoarthritis The Journal of the American Board of Family Medicine, 24 (2), 169-174 DOI: 10.3122/jabfm.2011.02.100156
- Homework Blood Pressure
Please write some notes about high and low blood pressure: What are the risks of high and low blood pressureHow can blood pressure be changed, for example how can high blood pressure be reduced?...
- #83 Question 6
The diagram below shows pressure changes in the left atrium and left ventricle of the heart and the aorta during the cardiac cycle. (a) Calculate how many heart beats there will be in one minute. (2 marks)(b) (i) On the diagram, indicate the point at...
- # 44 The Circulatory System - Blood Vessels
The mammalian circulatory system is a closed double circulation, consisting of a heart, blood vessels and blood. The heart produces high pressure --> blood moves through the vessels by mass flow. The mammalian...
- Arthrodesis
Term: arthrodesisLiterally meaning: ?joint binding?Origin: Anc Greek??????/arthron(=used as composing form pertaining to the joints eg Arhtropoda, arthritis etc)+?????(desis)= binding> ?????(dein)= to bindCoined/History(?)DefinitionArthrodesis ...
- Arthritis
Term: arthritis Origin: ?????/arthro(=joint) + -???? /it is(=inflammation) à disease of the joints Historical view: Archaeologists record that prehistoric man was often racked with arthritis and lamed by injuries. In Ancient Egypt, many skeletons had...
Biology
Gas, Knuckles, And The Little Blue Pill
Biology concepts ? dissolved gas, cavitation, arthritis, decompression sickness, ebulism, gas embolism
 |
It is certainly true that some folks love cracking their knuckles. The little research that has been conducted indicates that about 25-30% of people are habitual knuckle crackers, with the habit lasting on average 35 years. Fine for them, but we?re the ones who have to listen to it. |
This symphony of cracks and clicks makes you feel as though you can reach any key and can work faster and more dexterously than you could?ve before. Will you sound better now that you have cracked your knuckles? Nope ? you don?t play piano; you?re here to move it to the next room.
Question of the Day ? What makes the sound when you crack your knuckles and does it help or hurt you?
A knuckle is the joint where your carpal meets your metacarpal. A joint is any hinged meeting of two bones, made up of a sac (bursa) that keeps everything together and a small space between the bones (synovial space). Fluid in the space between the bones (synovial fluid) keeps the friction low as the bones flex in the joint.
Cracking joints can be done many at once or one at a time. Big joints can be cracked just as smaller joints. Chiropractic practices make a living out of cracking joints. There is an immediate feedback in hearing the joint crack; some treatment must have been rendered.
No matter how or why the joints are cracked, the production of the noise is the same in each case. Manipulation of the joint stretches the joint, separating the two bones, and creating a larger space than normal.
In physics, pressure is related to volume, being inversely related. The synovial joint has a certain amount of fluid, enough to fill the normal space. By increasing the joint space volume, the fluid will be filling a larger space. The same amount of fluid in a larger space means that the fluid will be under lower pressure.
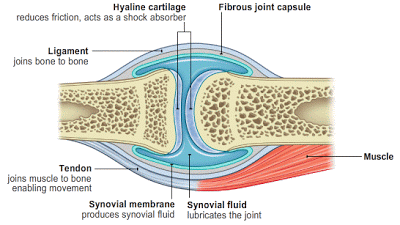 |
This is a generalized cartoon, but it describes the anatomy of most joints. The bursa is made up of the synovial membrane and the synovial fluid that buffers the joint. The bone ends are protected by the cartilage. The muscle, tendon, ligament and capsule hold the joint together. |
This decreased pressure in your closed joint will affect the dissolved gases in the synovial fluid. All fluids of the body, like blood or other extracellular fluid, even saliva, contains many molecules, including dissolved gases; CO2, N2, O2. Gases in solution are under pressure just like in their gaseous state. When the pressure decreases, the amount of gas that can stay in solution decreases (it becomes less soluble). When gas becomes insoluble, it comes out of solution and begins to form small bubbles (ebulism, a gas embolism). It is the formation of the bubble(s) that you hear.
Cavitation (formation of a cavity) in small joints wouldn't seem to be a high-energy event, and it isn?t, but it is enough to make the noise you hear. There is still some question as to how such a large noise can be made this way, but x-rays can show the presence of gas bubbles in the popped joint immediately after cracking.
After cracking your joint, the joint remains a little larger for a period of time, and slowly returns to its normal volume. During the time period that the joint is returning to the normal volume, pressure slowly increases. More pressure means more gas solubility, and the bubbles disappear. It takes a while, so you can?t crack that knuckle again for 15-20 minutes.
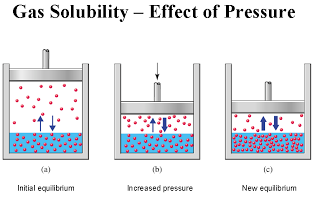 |
Here is a demonstration cartoon of pressure and gas solubility. An reduction in volume the volume from (a) to (b) causes an increase in pressure ? the same amount of stuff in a smaller space ? and this brings an increase in pressure and drives more gas into solution in (c). When popping your knuckle, we go the opposite direction, from (c) to (b), to (a), so less gas is soluble and bubbles will form in the synovial fluid. |
Is it bad for you to crack your joints? Does it do damage to your joints, either immediately or over time? There hasn?t been a lot of research done in this area, but what has been done shows that knuckle cracking does not lead to osteoarthritis.
A 2011 study is the most comprehensive done to date. These researchers looked at cracking and the frequency of cracking as well. No amount of cracking seemed to promoted arthritis development. A 1990 study stated that knuckle crackers were more likely to also have small amounts of hand inflammation and lower grip strength. However, this study could not conclude that knuckle cracking caused the inflammation or loss of grip strength.
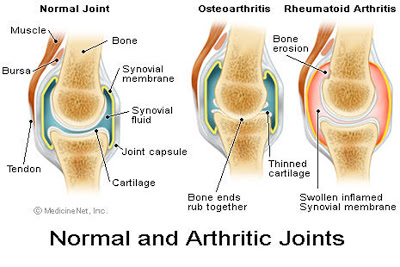 |
Arthritis comes from the Greek originally, where artho= joint, and itis = inflammation. So that's all arthritis is. There are two major forms, osteoarthritis is causes by a wearing down of the cartilage on the ends of the bone and a lass of the synovial buffer in between the bones. In rheumatoid arthritis, there is an autoimmune reaction where your body attacks its own joints and causes great inflammation. Knuckle cracking could only cause osteoarthritis, and it apparently doesn?t even do that. |
Oxygen?s counterpart, carbon dioxide, is also present in the blood, on its way back to the lungs to be exhaled. Most of the CO2is locked up as part of carbonic acid (H2CO3) or its conjugate base, bicarbonate (HCO3), and this helps to maintain the pH balance of your blood. Yet there is a little free CO2 dissolved in your blood as well.
The major dissolved gas in your blood is N2, nitrogen gas. Remember that air 80% nitrogen. This also happens to be the most soluble gas in your blood, so more of your body?s allotment of nitrogen is carried this way; less need for carrier molecules like hemoglobin or bicarbonate.
This is all well and good until the pressure on your body changes, like when you go scuba diving. Water weighs much more than air, so for every 10 m (33 ft) you descend in the water, the pressure on your body doubles. With more pressure, more gas will be soluble in the blood. This is the opposite reaction from when you stretch your joints while popping your knuckles.
The increased gas volume dissolved in your blood is no problem as long as you allow it to dissipate slowly. But if you have been at depth for some time, and then you ascend too quickly, your body doesn?t have time to adjust to the change in pressure.
The return to normal pressure means less gas will be soluble in your blood. Where is all the gas you added to your blood by diving deep going to go? It?s going to come out of solution and form bubbles. This is decompression sickness, sometimes called the bends.
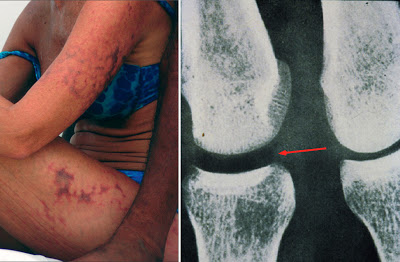 |
If you?re lucky, decompression sickness will only be as bad as the burst blood vessels in the skin shown on the left. Gas bubbles coming out of solution do damage to the endothelial cells that line the blood vessels, causing them to become leaky. Blood then spills into the tissues. On the right is what might happen when more gas comes out and starts to coalesce in the joint. The darker portion in the left joint is a gas bubble. The big red arrow should help you find the bubble. |
In the meantime, you are doubled over in pain (?the bends?). Pain is one thing, but if a bubble in your blood happens to get stuck somewhere, that?s called a gas embolus. Nothing downstream of the bubble is going to be getting oxygenated blood, and that means it will die. If it is in heart vessel, that causes a heart attack, if it?s in your lungs capillary beds, that?s a pulmonary embolism, if it is in your brain, that?s a stroke. Any of these can kill you.
The best way to treat the bends is to prevent them. You must ascend in stages, allowing time to adjust to the lower pressure at each depth. Your body will take the excess gas out of the blood if given time. When you learn to dive, much time is spent on the math involved in preventing decompression sickness; if you have been at such a depth for so long, you will need to ascend in X number of stages, with Y minutes at each stage depth.
If you don?t follow this, you?re in for a great deal of pain and a trip to a decompression chamber. In the chamber, they will pump in extra air to increase the pressure on your body, just like being at depth again. This will put the gas back into solution. Then they will release the pressure, a little at a time, allowing your body to take the excess gas out of your blood; the equivalent of a staged ascension for depth.
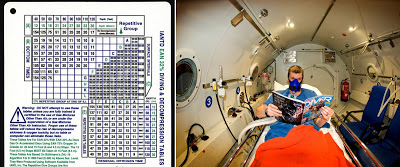 |
Here are your two choices to deal with recompression (reducing pressure as you ascend) when diving. On the left is a dive table, giving you the stages of your ascension needed to avoid the bends, according to your dive number, time, and depth. OR, you can ascend as fast as you like, and then spend 24 hours in the decompression chamber on the right. This is, of course, assuming you don?t die before they get you to the nearest chamber. |
Even pressurized airliners have lower than normal air pressure (your ears pop), so no one is advised to fly after diving at depth for at least 24 hours. Dives that don?t require a staged ascent should still be completed at least 12 hours before flying. If you go diving in the morning, get on a flight immediately afterward, and then start popping your knuckles ? could your hand explode?
Next week, another question in biology - can bacteria change the earth - the whole earth?
Blatteau, J., Brubakk, A., Gempp, E., Castagna, O., Risso, J., & Vallée, N. (2013). Sidenafil Pre-Treatment Promotes Decompression Sickness in Rats PLoS ONE, 8 (4) DOI: 10.1371/journal.pone.0060639
deWeber, K., Olszewski, M., & Ortolano, R. (2011). Knuckle Cracking and Hand Osteoarthritis The Journal of the American Board of Family Medicine, 24 (2), 169-174 DOI: 10.3122/jabfm.2011.02.100156
- Homework Blood Pressure
Please write some notes about high and low blood pressure: What are the risks of high and low blood pressureHow can blood pressure be changed, for example how can high blood pressure be reduced?...
- #83 Question 6
The diagram below shows pressure changes in the left atrium and left ventricle of the heart and the aorta during the cardiac cycle. (a) Calculate how many heart beats there will be in one minute. (2 marks)(b) (i) On the diagram, indicate the point at...
- # 44 The Circulatory System - Blood Vessels
The mammalian circulatory system is a closed double circulation, consisting of a heart, blood vessels and blood. The heart produces high pressure --> blood moves through the vessels by mass flow. The mammalian...
- Arthrodesis
Term: arthrodesisLiterally meaning: ?joint binding?Origin: Anc Greek??????/arthron(=used as composing form pertaining to the joints eg Arhtropoda, arthritis etc)+?????(desis)= binding> ?????(dein)= to bindCoined/History(?)DefinitionArthrodesis ...
- Arthritis
Term: arthritis Origin: ?????/arthro(=joint) + -???? /it is(=inflammation) à disease of the joints Historical view: Archaeologists record that prehistoric man was often racked with arthritis and lamed by injuries. In Ancient Egypt, many skeletons had...
