Biology
 All living organisms need a continuous supply of energy to maintain their metabolism. They must absorb either light energy in photosynthesis or chemical potential energy to do the work necessary to stay alive.
All living organisms need a continuous supply of energy to maintain their metabolism. They must absorb either light energy in photosynthesis or chemical potential energy to do the work necessary to stay alive.
Such work includes:
? Anabolic reactions: the synthesis of proteins and other large molecules from smaller ones (e.g. polypeptides from amino acids)
? Active transport of ions and molecules across cell membranes against their concentration gradient (e.g. the activity of the sodium?potassium pumpneed energy as against concentration gradient)
? Movement (mechanical work): movement of the whole organism for example muscle contraction (such as heart beat, breathing movements, walking) or movement within the organism, e.g. movement of organelles in cells)
? Maintenance of body temperature, particularly in mammals and birds, which must release thermal energy to maintain the body temperature above that of the environment.
? Transmission of nerve impulses.
Photosynthesis transfers light energy into chemical potential energy, which can then be released for work by the process of respiration. Both photosynthesis and respiration involve an important intermediary molecule in this energy transfer: adenosine triphosphate (ATP). In many living organisms, most of the energy transferred to ATP is derived originally from light energy; a few prokaryotes (the chemoautotrophs) are not dependent on light energy trapped by photosynthesis but use energy from inorganic chemical reactions instead.
ATP
1. Structure

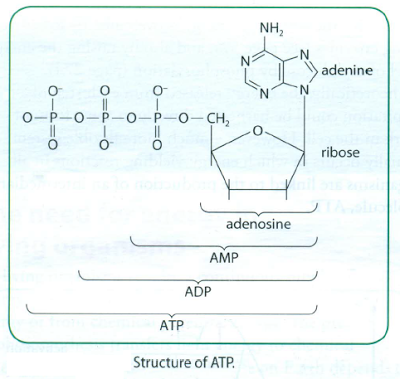
ATP consists of the organic base adenine and the pentose sugar ribose. Together these make the nucleoside adenosine. This is combined with three phosphate groups. ATP is therefore an activated phosphorylated nucleotide.
2. The universal energy currency
Processes in cells that require energy are linked to chemical reactions that yield energy by an intermediary molecule, ATP. Using one type of molecule to transfer energy to many different energy-requiring processes makes it easier for these processes to be controlled and coordinated. All organisms use ATP as their energy currency: it is a universal energy currency. ATP is never stored. Glucose and fatty acids are short-term energy stores, while glycogen, starch and triglycerides are long-term stores.
When an ATP molecule is hydrolysed, losing one of its phosphate groups, some of this energy is released and can be used by the cell. In this process, the ATP is converted to ADP (adenosine d iphosphate). The hydrolysis of one ATP molecule releases a small 'packet' of energy that is often just the right size to fuel a particular step in a process. A glucose molecule, on the other hand, would contain far too much energy, so a lot would be wasted if cells used glucose molecules as their immediate source of energy.
ATP can be synthesised from ADP and an inorganic phosphate group (Pi) using energy, and hydrolysed to ADP and phosphate to release energy. This interconversion is all-important in providing energy for a cell. Hydrolysis of the terminal phosphate group of ATP releases 30.5 kJ mol?1 of energy for cellular work:

Removing the second phosphate, giving AMP, also releases 30.5 kJ mol?1 of energy, but removing the last phosphate yields only 14.2 kJ mol?1. The energy released comes not simply from these bonds, but from the chemical potential energy of the molecule as a whole.

Each cell has only a tiny quantity of ATP in it at any one time. The cell does not import ATP, adenosine diphosphate (ADP) or adenosine monophosphate (AMP). With few exceptions, each cell must produce its own ATP by respiration and recycle it very rapidly. Because it is a small, watersoluble molecule, it is easily moved from where it is made in a cell to where it is needed.
3. The roles
? binding to a protein molecule, changing its shape and causing it to fold differently, to produce movement, e.g. muscle contraction
? binding to an enzyme molecule, allowing an energy-requiring reaction to be catalysed
? transferring a phosphate group to an enzyme, making the enzyme active
? transferring a phosphate group to an unreactive substrate molecule so that it can react in a specific way, e.g. in glycolysis and the Calvin cycle
? transferring AMP to an unreactive substrate molecule, producing a reactive intermediate compound, e.g. amino acids before binding to tRNA during protein synthesis
? binding to a trans-membrane protein so that active transport can take place across the membrane.
- Catabolic And Anabolic
What exactly are Catabolic and Anabolic reactions?Catabolic and anabolic reactions are both metabolic processes that go to support an organisms metabolism. Catabolic reactions make energy for the cell to use by breaking down large molecules...
- #97 Summary Of Energy And Respiration
1 Organisms must do work to stay alive. The energy input necessary for this work is either light, for photosynthesis, or the chemical potential energy of organic molecules. Work includes anabolic reactions, active transport and movement. Some organisms,...
- #87 Respiration, Glycolysis
Respiration is the oxidation of energy-containing organic molecules. The energy released from this process is used to combine ADP with inorganic phosphate to make ATP. All cells obtain useable energy through respiration. Most cells use carbohydrate,...
- #85 Energy And Respiration - Syllabus 2016
12.1 Energy12.2 Respiration Energy is a fundamental concept in biology. All living things require a source of cellular energy to drive their various activities. ATP is the universal energy currency as its molecules are small,...
- Photosynthesis And Cellular Respiration
Photosynthesis is the processes of converting carbon dioxide and water, using light energy, into glucose and oxygen. Plants, algae, and certain prokaryotes capture about 1% of the energy in the sunlight that reaches Earth and convert it to chemical energy...
Biology
#86 Energy and ATP

Such work includes:
? Anabolic reactions: the synthesis of proteins and other large molecules from smaller ones (e.g. polypeptides from amino acids)
? Active transport of ions and molecules across cell membranes against their concentration gradient (e.g. the activity of the sodium?potassium pumpneed energy as against concentration gradient)
? Movement (mechanical work): movement of the whole organism for example muscle contraction (such as heart beat, breathing movements, walking) or movement within the organism, e.g. movement of organelles in cells)
? Maintenance of body temperature, particularly in mammals and birds, which must release thermal energy to maintain the body temperature above that of the environment.
? Transmission of nerve impulses.
Photosynthesis transfers light energy into chemical potential energy, which can then be released for work by the process of respiration. Both photosynthesis and respiration involve an important intermediary molecule in this energy transfer: adenosine triphosphate (ATP). In many living organisms, most of the energy transferred to ATP is derived originally from light energy; a few prokaryotes (the chemoautotrophs) are not dependent on light energy trapped by photosynthesis but use energy from inorganic chemical reactions instead.
ATP
1. Structure

ATP consists of the organic base adenine and the pentose sugar ribose. Together these make the nucleoside adenosine. This is combined with three phosphate groups. ATP is therefore an activated phosphorylated nucleotide.

2. The universal energy currency
Processes in cells that require energy are linked to chemical reactions that yield energy by an intermediary molecule, ATP. Using one type of molecule to transfer energy to many different energy-requiring processes makes it easier for these processes to be controlled and coordinated. All organisms use ATP as their energy currency: it is a universal energy currency. ATP is never stored. Glucose and fatty acids are short-term energy stores, while glycogen, starch and triglycerides are long-term stores.
When an ATP molecule is hydrolysed, losing one of its phosphate groups, some of this energy is released and can be used by the cell. In this process, the ATP is converted to ADP (adenosine d iphosphate). The hydrolysis of one ATP molecule releases a small 'packet' of energy that is often just the right size to fuel a particular step in a process. A glucose molecule, on the other hand, would contain far too much energy, so a lot would be wasted if cells used glucose molecules as their immediate source of energy.
 |
Hydrolysis of ATP. |

Removing the second phosphate, giving AMP, also releases 30.5 kJ mol?1 of energy, but removing the last phosphate yields only 14.2 kJ mol?1. The energy released comes not simply from these bonds, but from the chemical potential energy of the molecule as a whole.

Each cell has only a tiny quantity of ATP in it at any one time. The cell does not import ATP, adenosine diphosphate (ADP) or adenosine monophosphate (AMP). With few exceptions, each cell must produce its own ATP by respiration and recycle it very rapidly. Because it is a small, watersoluble molecule, it is easily moved from where it is made in a cell to where it is needed.
3. The roles
? binding to a protein molecule, changing its shape and causing it to fold differently, to produce movement, e.g. muscle contraction
? binding to an enzyme molecule, allowing an energy-requiring reaction to be catalysed
? transferring a phosphate group to an enzyme, making the enzyme active
? transferring a phosphate group to an unreactive substrate molecule so that it can react in a specific way, e.g. in glycolysis and the Calvin cycle
? transferring AMP to an unreactive substrate molecule, producing a reactive intermediate compound, e.g. amino acids before binding to tRNA during protein synthesis
? binding to a trans-membrane protein so that active transport can take place across the membrane.
ATP made, moved around and used in most cells: ? ATP produced using energy from respiration reactions ? Breaks down to release energy when required ATP? ADP + Pi + energy ? It is an immediate source of energy released in small ?packets? ? Rapid turnover of ATP with anabolic and catabolic processes ? Uses eg. active transport/Na pump/cell division/phosphorylation |
Metabolism
The total of all the biochemical reactions needed for an organism to stay alive is its
metabolism.
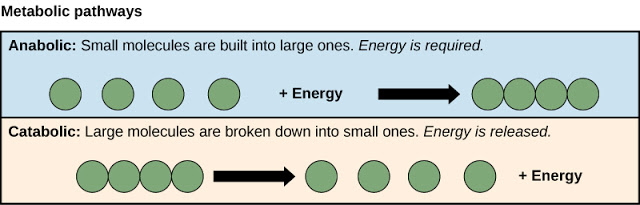
metabolism = anabolism + catabolism
Anabolism is the building up of more complex molecules from simpler ones, for example the synthesis of nucleic acids and carbohydrates. Enzymes are needed for these syntheses of the complex molecules needed for growth. Anabolic reactions are energy-consuming.
Catabolism is the enzymic breakdown of complex molecules to simpler ones. It is the opposite of anabolism. The catabolic reactions of respiration yield energy.

Syllabus: 12.1 Energy ATP is the universal energy currency as it provides the immediate source of energy for cellular processes. a) outline the need for energy in living organisms, as illustrated by anabolic reactions, such as DNA replication and protein synthesis, active transport, movement and the maintenance of body temperature b) describe the features of ATP that make it suitable as the universal energy currency |
- Catabolic And Anabolic
What exactly are Catabolic and Anabolic reactions?Catabolic and anabolic reactions are both metabolic processes that go to support an organisms metabolism. Catabolic reactions make energy for the cell to use by breaking down large molecules...
- #97 Summary Of Energy And Respiration
1 Organisms must do work to stay alive. The energy input necessary for this work is either light, for photosynthesis, or the chemical potential energy of organic molecules. Work includes anabolic reactions, active transport and movement. Some organisms,...
- #87 Respiration, Glycolysis
Respiration is the oxidation of energy-containing organic molecules. The energy released from this process is used to combine ADP with inorganic phosphate to make ATP. All cells obtain useable energy through respiration. Most cells use carbohydrate,...
- #85 Energy And Respiration - Syllabus 2016
12.1 Energy12.2 Respiration Energy is a fundamental concept in biology. All living things require a source of cellular energy to drive their various activities. ATP is the universal energy currency as its molecules are small,...
- Photosynthesis And Cellular Respiration
Photosynthesis is the processes of converting carbon dioxide and water, using light energy, into glucose and oxygen. Plants, algae, and certain prokaryotes capture about 1% of the energy in the sunlight that reaches Earth and convert it to chemical energy...
