Biology
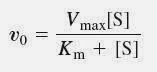 Michaelis-Menten equation describes the velocity of enzymatic reactions (v) by relating it to [S] - concentration of a substrate S.
Michaelis-Menten equation describes the velocity of enzymatic reactions (v) by relating it to [S] - concentration of a substrate S.
Michaelis - Menten Equation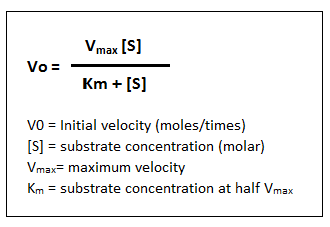
- Enzyme Inhibition
In competitive inhibition, inhibitor has a shape, charge, size and structure similar to that of the substrate. It therefore competes with the substrate for the active sites to form the enzyme-inhibitor complex (E-I complex) and reduces the number of active...
- #20.factors Affecting The Rate Of Enzyme-catalysed Reactions
These factors are: - Temperature - pH - Enzyme concentration - Substrate concentration - Inhibitor concentration When an enzyme solution is added to a solution of its substrate, the molecules collide. With time, the quantity of substrate...
- #18. Enzymes - Active Site, Activation Energy, Enzyme Specificity
Enzymes are globular proteins that serve as biological catalysts. They speed up or slow down metabolic reaction, but remain unchanged. They may facilitate the breaking of an existing bond or the formation of a new bond....
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
- Enzymes
ENZYMES Enzymes are biological catalysts which influence biochemical reactions. All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes. Ribozymes: These are the Nucleic acids (RNA) that behave like...
Biology
# 21 Michaelis - Menten Equation and Immobilising an enzyme
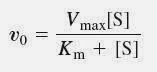
Michaelis - Menten Equation

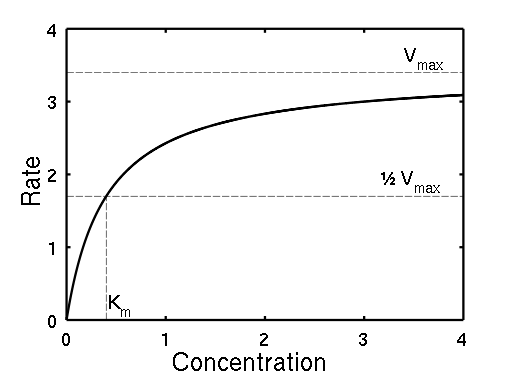 |
| An example curve with parametersVmax = 3.4 and Km = 0.4. Wikipedia. |
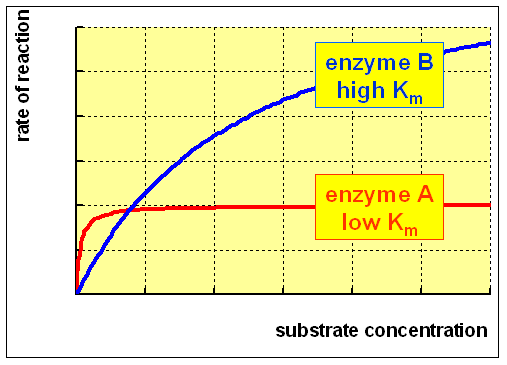
Here, Vmax represents the maximum rate achieved by the system, at maximum (saturating) substrate concentrations.
The Michaelis constant Km is the substrate concentration at which the reaction rate is half of Vmax.
Km is (roughly) an inverse measure of the affinity or strength of binding between the enzyme and its substrate. The lower the Km, the greater the affinity (so the lower the concentration of substrate needed to achieve a given rate).
It permits prediction of whether or not the rate of formation of product will be affected by the availability of substrate.
 |
| ucl.ac.uk |
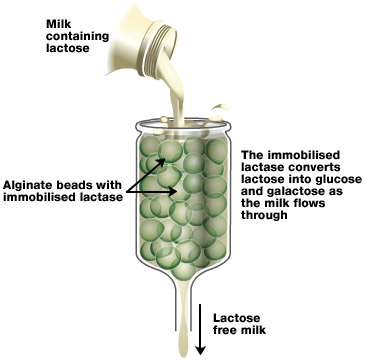
Immobilising an enzyme in alginate
? In industry enzymes are used on a large scale.
? It is very costly to use enzymes only once, but most enzymes are only commercially available in liquid or dehydrated forms and once they have been used in solution it is very difficult and time consuming to separate them from the product.
? To allow their re-use, enzymes may be immobilised (attached to an inert, insoluble material such as calcium alginate to form a gel capsule around them). This way, the enzymes will be hold in place throughout the reaction, easily separated from the products and may be used again.
? One way of immobilising enzymes: mixing the enzyme with sodium alginate, then dropping the mixture into calcium chloride solution. Sodium alginate will turn from liquid to solid when immersed in calcium chloride. This produces small beads with the enzyme inside.
2 Na(Alginate) + Ca++ -------> Ca(Alginate)2 + 2 Na+
? The substrate that the enzyme acts upon is able to diffuse through the gel, although this may be quite slow.
? Immobilised enzymes are widely used in industry because it allows the reaction to flow continuously and the product will not be contaminated with the enzyme so will not need to be purified.

Syllabus 2016 Factors that affect enzyme action b) explain that the maximum rate of reaction (Vmax) is used to derive the Michaelis-Menten constant (Km) which is used to compare the affinity of different enzymes for their substrates d) investigate and explain the effect of immobilising an enzyme in alginate on its activity as compared with its activity when free in solution. |
- Enzyme Inhibition
In competitive inhibition, inhibitor has a shape, charge, size and structure similar to that of the substrate. It therefore competes with the substrate for the active sites to form the enzyme-inhibitor complex (E-I complex) and reduces the number of active...
- #20.factors Affecting The Rate Of Enzyme-catalysed Reactions
These factors are: - Temperature - pH - Enzyme concentration - Substrate concentration - Inhibitor concentration When an enzyme solution is added to a solution of its substrate, the molecules collide. With time, the quantity of substrate...
- #18. Enzymes - Active Site, Activation Energy, Enzyme Specificity
Enzymes are globular proteins that serve as biological catalysts. They speed up or slow down metabolic reaction, but remain unchanged. They may facilitate the breaking of an existing bond or the formation of a new bond....
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
- Enzymes
ENZYMES Enzymes are biological catalysts which influence biochemical reactions. All enzymes are proteins but all proteins are not enzymes because there are proteins other than enzymes. Ribozymes: These are the Nucleic acids (RNA) that behave like...
