Biology
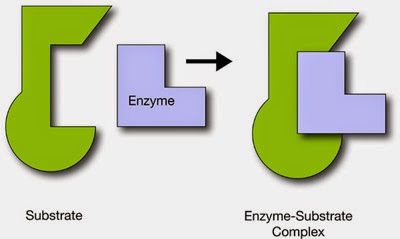 1 Enzymes are globular proteins which catalyse metabolic reactions.
1 Enzymes are globular proteins which catalyse metabolic reactions.
2 Each enzyme has an active site with a specific shape, into which the substrate molecule or molecules fit precisely. This is the lock and key hypothesis ? the substrate is compared with a key which fits precisely into the lock of the enzyme.
3 The lock and key hypothesis has been modified. The modern hypothesis is called the induced fit
hypothesis. The active site is no longer regarded as a rigid structure like a lock, but as a flexible structure which can change shape slightly to fit precisely the substrate molecule.
4 When the substrate enters the active site, an enzyme?substrate complex is temporarily formed in which the R groups of the amino acids in the enzyme hold the substrate in place.
5 Enzymes may be involved in reactions which break down molecules or join molecules together.
6 Enzymes work by lowering the activation energy of the reactions they catalyse.
7 The course of an enzyme reaction can be followed by measuring the rate at which a product is formed or the rate at which a substrate disappears. A progress curve, with time on the x-axis, can be plotted. The curve is steepest at the beginning of the reaction, when substrate concentration is at its highest. This rate is called the initial rate of reaction.
8 Various factors aff ect the rate of activity of enzymes. Four important factors are enzyme concentration, substrate concentration, temperature and pH.
9 Th e greater the concentration of the enzyme, the faster the rate of reaction, provided there are enough
substrate molecules present. Similarly, the greater the concentration of the substrate, the faster the rate
of reaction, provided enough enzyme molecules are present. During enzyme reactions, rates slow down
as substrate molecules are used up.
10 Each enzyme has an optimum temperature at which it works fastest. As temperature increases above
the optimum temperature, the enzyme gradually denatures (loses its precise tertiary structure). When
it is completely denatured, it ceases to function. Denaturation is sometimes reversible.
11 Each enzyme has an optimum pH. Some enzymes operate within a narrow pH range; some have a
broad pH range.
12 Enzymes are also aff ected by the presence of inhibitors, which slow down their rate of reaction or
stop it completely.
13 Competitive inhibitors are molecules which are similar in shape to the normal substrate molecules.
Th ey compete for the active site of the enzyme. This type of inhibition is reversible because the inhibitor can enter and leave the active site.
14 Non-competitive inhibitors either bind permanently to the active site or bind at a site elsewhere on the enzyme, causing a change in shape of the active site. Such binding may or may not be reversible.
Multiple - choise Test
1 Which of the following describes an enzyme?
A a catalyst with an active site which binds to the product of a reaction
B a fibrous protein with an active site which binds to a substrate
C a globular protein with hydrophilic groups on its surface
D an insoluble biological catalyst
2 The graph shows the energy changes during the progress of a chemical reaction.
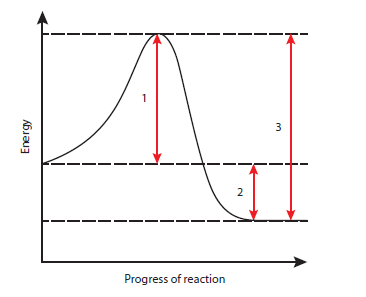
Which of the energy changes could be decreased by adding an enzyme?
A 1, 2 and 3
B 1 and 2 only
C 1 and 3 only
D 2 and 3 only
3 Which tests, carried out on samples taken at intervals during the course of the reaction below, would enable the progress of the reaction to be followed?

1 Benedict?s test
2 biuret test
3 emulsion test
4 iodine in potassium iodide solution test
A 1 or 2
B 1 or 4
C 2 or 3
D 3 or 4
4 Which of the following describes the effects of temperature on an enzyme-controlled reaction?
A At low temperatures, substrate molecules only rarely collide with an enzyme?s active site.
B At low temperatures, the enzyme loses its shape and activity.
C At low temperatures, the enzyme becomes denatured.
D As the temperature increases, the number of collisions between enzyme and substrate decrease.
5 Which statement does not describe the effect on an enzyme?s activity of changing the pH.
A A pH that is very different from the enzyme?s optimum pH can denature the enzyme.
B At low pH there are fewer hydrogen ions to interact with the R groups of the amino acids that make up the enzyme.
C Changing the pH alters the interactions of the amino acids that make up the enzyme.
D Changing the pH alters the enzyme?s three-dimensional shape.
6 The graph shows the effect of increasing the substrate concentration on the rate of an enzyme-catalysed reaction.
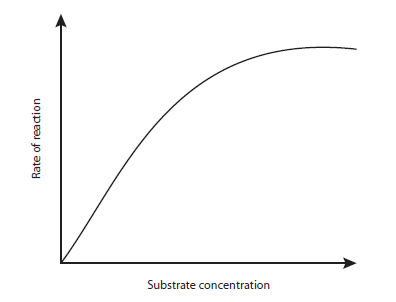
What explains why the rate of reaction levels off?
A All the enzyme active sites are saturated with substrate.
B All the substrate has been used up.
C The concentration of the enzyme is gradually decreasing.
D The rate at which enzyme and substrate collide is decreasing.
7 Curve X shows the progress of an enzyme-catalysed reaction under optimum conditions.
Curve Y shows the same reaction with one factor changed throughout the reaction.
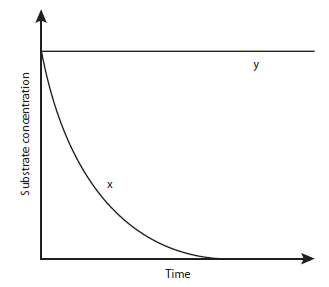
Which factor was changed?
A A competitive inhibitor was added.
B A non-competitive inhibitor was added.
C The reaction took place at a different temperature.
D The reaction took place at a different pH.
8 These statements are about enzyme inhibitors.
1 binds reversibly to an enzyme
2 binds to a site on the enzyme different from the active site
3 has a structural similarity to the enzyme?s normal substrate
4 can be displaced from the enzyme by a high concentration of the enzyme?s normal substrate
Which statements describe a competitive inhibitor?
A 1, 2, 3 and 4
B 1, 3 and 4 only
C 2 and 3 only
D 3 and 4 only
9 Fibrous protein from dead cells is difficult to remove from contact lenses. Some cleaning solutions contain an enzyme to digest this protein to soluble products.
What describes the enzyme and its activity?
A An active site on a fibrous protein binds to the enzyme and is hydrolysed.
B An active site on a soluble product binds to the enzyme and is digested.
C An active site on a globular protein binds to a soluble product and digests it.
D An active site on a globular protein binds to a fibrous protein and hydrolyses it.
10 Several different organisms produce lactase enzymes to hydrolyse lactose. The different enzymes have different molecular sizes.
Which description of these different lactases is correct?
A Their active sites have the same shape.
B Their primary structure is the same.
C They each contain the same number of amino acids.
D They have exactly the same three-dimensional structure.
Answer for Multiple - choise Test
1 C
2 C
3 B
4 A
5 B
6 A
7 B
8 B
9 D
10 A
End-of-chapter questions
1 The diagram below shows an enzyme and an inhibitor of the enzyme. Which of the following describes the inhibitor?
A competitive, irreversible B competitive, reversible
C non-competitive, irreversible
D non-competitive, reversible

2 In a reaction controlled by an enzyme, which of the following graphs shows the effect of substrate concentration on the rate of the reaction?
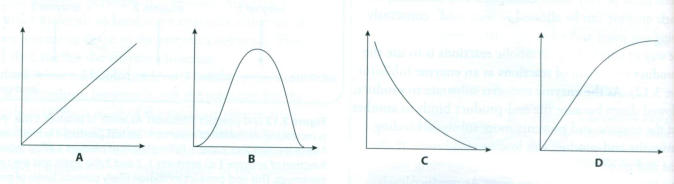
 3 The graph shows the progress of the digestion of starch by the enzyme salivary amylase. Why does the reaction slow down?
3 The graph shows the progress of the digestion of starch by the enzyme salivary amylase. Why does the reaction slow down?
A End-product inhibition by maltose.
B The salivary amylase is becoming denatured.
C The salivary amylase is gradually becoming saturated with starch.
D There are fewer and fewer substrate molecules left to bind with the salivary amylase.
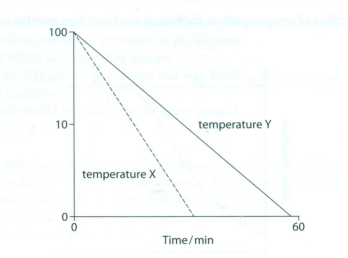
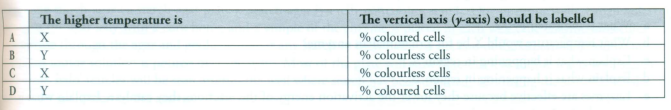
4 If methylene blue dye is added to a suspension of yeast cells, living cells do not take up the stain, and they remain colourless. However, dead cells are stained blue.This fact was used to carry out an investigation into the rate at which yeast cells were killed at two different temperatures (at high temperatures the yeast enzymes will be denatured).

a Describe the effect of pH on this enzyme.
b Explain why pH affects the activity of the enzyme.
7 The graph below shows the effect of temperature on the rate of reaction of an enzyme.
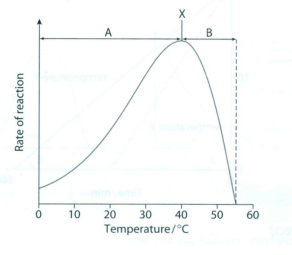
a What is indicated by X?
b What temperature would X be for a mammalian enzyme?
c Explain what is happening in region A.
d Explain what is happening in region B.
e Enzymes are effective because they lower the activation energy of the reactions they catalyse. Explain what is meant by 'activation energy'.
8 The reaction below occurs during aerobic respiration. The reaction is catalysed by the enzyme succinic dehydrogenase.

a Name the substrate in the above reaction. [1] b The molecule malonic acid, which is shown below, inhibits this reaction. It does not bind permanently to the enzyme. Describe how malonic acid inhibits the enzyme succinic dehydrogenase. [3]

c Heavy metals such as lead and mercury bind permanently to -SH groups of amino acids present in enzymes. These -SH groups could be in the active site or elsewhere in the enzyme.
i Name the amino acid which contains -SH groups.[1]
ii Explain the function of -SH groups in proteins and why binding of heavy metals to these groups would inhibit the activity of an enzyme.[4]
iii What type of inhibition would be caused by the heavy metals?[1]
9. You are provided with three solutions: A, B and C. One solution contains the enzyme amylase, one contains starch andone contains glucose. Starch is the substrate of the enzyme. The product is the sugar maltose. You are provided withonly one reagent, Benedict's solution, and the usual laboratory apparatus.
a Outline the procedure you would follow to identify the three solutions.[6]
b What type of reaction is catalysed by the enzyme? [1]
10 The activity of the enzyme amylase can be measured at a particular temperature by placing a sample into a Petri dish containing starch-agar ('a starch-agar plate'). Starch-agar is a jelly containing starch. One or more 'wells' (small holes) arecut in the agar jelly with a cork borer, and a sample of the enzyme is placed in each well. The enzyme molecules then diffuse through the agar and gradually digest any starch in their path. At the end of the experiment, iodine in potassium iodide solution is poured over the plate. Most of the plate will turn blue-black as iodine reacts with starch, but a clear 'halo' (circle) will be seen around the well where starch has been digested. Measuring the size of the halo cangive an indication of the activity of the enzyme.
A student decided to investigate the rate at which a mammalian amylase is denatured at 60°C. She heated different amples of the enzyme in a water bath at 60°C for 0, 1, 5, 10 and 30 minutes. She then allowed the samples to cool downto room temperature and placed samples of equal volume in the wells of five starch-agar plates, one plate for eachheating period. She then incubated the plates in an oven at 40°C for 24 hours.
The results of the student's experiment are shown on the next page. A diagram of one dish is shown, and the real size of one halo from each dish is also shown.
a Why did the student cut four wells in each dish rather than just one? [1]
b One dish contained samples from amylase which was not heated (time zero). This is a control dish. Explain the purpose of this control. [1]
c Explain why the starch-agar plates were incubated at 40°C and not room temperature. [1]
d Describe what was happening in the dishes during the 24 hours of incubation [4]
e Why was it important to add the same volume of amylase solution to each well? [1]
f Measure the diameter in mm of the representative halo from each dish. Record the results in a suitable table. [4]
g Only one halo from each dish is shown in the diagrams. In practice there was some variation in the diameters of the four halos in each dish. How would you allow for this when processing your data? [1]
h Plot a graph to show the effect of length of time at 60°C on the activity of the enzyme. [5]
i Describe and explain your results. [4]
j Another student discovered that amylases from fungi and bacteria are more resistant to high temperatures than mmalian amylases. Using starch-agar plates as a method for measuring the activity of an amylase at 40°C, outline an experiment that the student could perform to discover which amylase is most resistant to heat. Note that temperatures up to 120 °C can be obtained by using an autoclave (pressure cooker).
k Enzymes are used in many industrial processes where resistance to high temperatures is an advantage.[5]
State three other variables apart from temperature which should be controlled in an industrial process involving enzymes.[3]

Answer for end-of-chapter
1 B
2 D
3 D
4 C
5 straight line drawn from origin at zero to show steepest gradient of curve;
6 a maximum activity/optimum pH, is pH 5; activity gradually increases between pH 2 and
pH 5, and decreases from pH 5 to pH 10; activity very low at pH 2 and pH 10;
b pH is a measure of the hydrogen ion concentration; hydrogen ions are positively charged;
hydrogen ions can interact with the R groups of amino acids; affects ionic bonding/affects ionisation of R groups; affects tertiary structure/affects 3D shape of enzyme; therefore substrate may not fit active site (as precisely);
7 a optimum temperature;
b 37 °C accept 40 °C
c as temperature increases the kinetic energy of the molecules increases; the rate of collision between substrate and, enzyme/active site, increases; rate of reaction increases;
d the enzyme is gradually being denatured; when the rate is zero the enzyme is completely
denatured; enzyme loses tertiary structure; substrate no longer fits into active site/active site
loses its (specific) shape so substrate does not fit; hydrogen bonds broken/increased vibration of
enzyme molecule;
e the extra energy which must be given to the substrate; before it can be converted into the product;
Exam-style questions
8 a succinic acid; [1]
b malonic acid acts as a competitive inhibitor; it has a similar shape/structure to succinic acid;
it therefore competes with succinic acid for a place in the active site of the enzyme; [3]
c i cysteine; [1]
ii ?SH groups form disulfi de bridges; used to determine tertiary structure; heavy metal would prevent formation of disulfi de bridges; could change shape of active site; heavy metal could affect shape either by binding directly in the active site, or by binding at another site which then results in change in shape of the active site; substrate would not be able to fi t into active site; [max. 4]
iii (non-competitive) irreversible; [1]
b malonic acid acts as a competitive inhibitor;
it has a similar shape/structure to succinic acid;
it therefore competes with succinic acid for a place in the active site of the enzyme; [3]
c i cysteine; [1]
ii ?SH groups form disulfi de bridges;
used to determine tertiary structure;
heavy metal would prevent formation of disulfi de bridges;
could change shape of active site;
heavy metal could aff ect shape either by binding directly in the active site, or by binding at another site which then results in change in shape of the active site; substrate would not be able to fit into active site; [max. 4]
iii (non-competitive) irreversible; [1]
a positive result/brick-red precipitate will be seen, with the glucose solution;
heat separate samples of the two remaining solutions, in boiling water bath/to high
temperature (e.g. 80 °C), for suitable time/at least two minutes (enzyme will be denatured);
for each heated solution, mix it with an unheated sample of the other solution;
leave several minutes/suitable time (for reaction to take place);
carry out Benedict?s test on the two tubes;
only one will give a positive result (due to presence of maltose) and this will be the one
which contained the unheated enzyme;
accept alternative wording for all steps in the procedure, provided the same logical sequence
is described [max. 6]
b hydrolysis; [1]
b to act as a reference to show what happens if there is no denaturation; AW [1]
c 40 °C is the optimum temperature for a mammalian enzyme; [1]
d enzyme/amylase (molecules) diff use(s) from wells into the agar;
enzyme/amylase digests the starch;
to maltose;
forms rings/halos, of digested starch around the wells;
amount of digestion/rate of digestion, is related to degree of denaturation of enzyme/amylase;[max. 4]
e the more enzyme/amylase added, the greater the amount of digestion of starch
or
want results to be due to diff erences in preheating times, not to diff erences in amount of amylase/enzyme; AW [1]
f
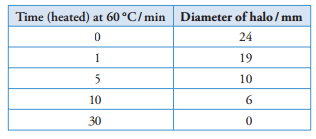
table drawn with ruled lines for border and to separate columns and headings (ideally ruled lines
between rows, but not essential for mark);
correct headings to columns with units;
first column is independent variable (Time heated at 60 °C);
correct measurements of halos; [4]
g measure the four halos and calculate the mean; [1]
(any anomalous results should be ignored)
h
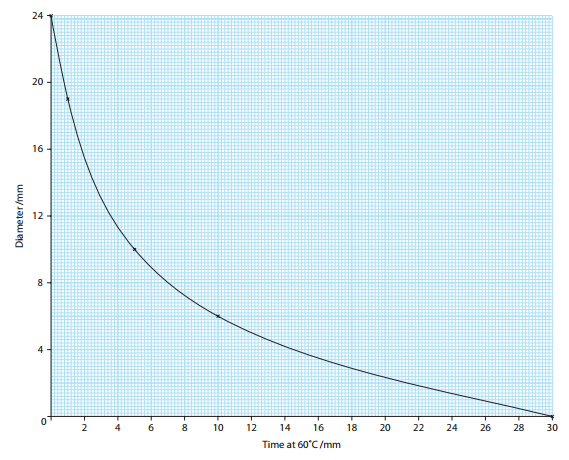 x-axis (horizontal axis) is labelled ?Time (heated) at 60 °C?, y-axis (vertical axis) is labelled ?Diameter?;
x-axis (horizontal axis) is labelled ?Time (heated) at 60 °C?, y-axis (vertical axis) is labelled ?Diameter?;
units given on axes, min/minutes and mm;
regular intervals on both axes (check that 0, 1, 5, 10, 30 are not regularly spaced on x-axis);
points plotted accurately;
points joined with straight lines or smooth curve; [5]
i enzyme was completely denatured after 30 minutes;
rate of denaturation was rapid at first and then gradually slowed down;
data quoted;
enzyme loses tertiary structure;
substrate no longer fi ts into active site/active site loses its (specific) shape so substrate does not fit;
AVP e.g. hydrogen bonds broken/increased vibration of enzyme molecule; [max. 4]
j heat samples of mammalian, fungal and bacterial amylases at diff erent temperatures;
suitable range, e.g. between 40 °C and 120 °C;
40 °C is a control (for reference to fi nd out size of halo with no denaturation);
at least five temperatures, e.g. 40, 60, 80, 100, 120 °C;
heat for suitable length of time (e.g. one hour, at least ten min);
cool to room temp/40 °C, add equal volumes to wells in starch?agar plates, replicate wells in each
plate (e.g. four), leave 24 hours, test for starch, measure diameters of halos; [max. 5]
Background information: amylase enzymes from the bacterium Bacillus licheniformis and
the fungus Aspergillus have been developed by biotechnology companies for use in industrial
processes. For example, a bacterial amylase that functions in the range 90?110 °C has been
developed and is used in beer brewing and other processes, and a fungal amylase that operates in
the range 50?60 °C is used for pastry baking and maltose syrup production.
k pH ;
substrate concentration;
enzyme concentration; [3]
- Q: Explain The Effects Of Too High A Temperature On An Enzyme
If the temperature is too high, an increase in kinetic energy of the molecules will cause excessive vibrations of the molecules. This will disrupt the 3 dimensional structure of the enzyme, causing it to become a random coil. The enzyme is therefore denatured....
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #20.factors Affecting The Rate Of Enzyme-catalysed Reactions
These factors are: - Temperature - pH - Enzyme concentration - Substrate concentration - Inhibitor concentration When an enzyme solution is added to a solution of its substrate, the molecules collide. With time, the quantity of substrate...
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
Biology
#22 Summary of Enzymes

2 Each enzyme has an active site with a specific shape, into which the substrate molecule or molecules fit precisely. This is the lock and key hypothesis ? the substrate is compared with a key which fits precisely into the lock of the enzyme.
3 The lock and key hypothesis has been modified. The modern hypothesis is called the induced fit
hypothesis. The active site is no longer regarded as a rigid structure like a lock, but as a flexible structure which can change shape slightly to fit precisely the substrate molecule.
4 When the substrate enters the active site, an enzyme?substrate complex is temporarily formed in which the R groups of the amino acids in the enzyme hold the substrate in place.
5 Enzymes may be involved in reactions which break down molecules or join molecules together.
6 Enzymes work by lowering the activation energy of the reactions they catalyse.
7 The course of an enzyme reaction can be followed by measuring the rate at which a product is formed or the rate at which a substrate disappears. A progress curve, with time on the x-axis, can be plotted. The curve is steepest at the beginning of the reaction, when substrate concentration is at its highest. This rate is called the initial rate of reaction.
8 Various factors aff ect the rate of activity of enzymes. Four important factors are enzyme concentration, substrate concentration, temperature and pH.
9 Th e greater the concentration of the enzyme, the faster the rate of reaction, provided there are enough
substrate molecules present. Similarly, the greater the concentration of the substrate, the faster the rate
of reaction, provided enough enzyme molecules are present. During enzyme reactions, rates slow down
as substrate molecules are used up.
10 Each enzyme has an optimum temperature at which it works fastest. As temperature increases above
the optimum temperature, the enzyme gradually denatures (loses its precise tertiary structure). When
it is completely denatured, it ceases to function. Denaturation is sometimes reversible.
11 Each enzyme has an optimum pH. Some enzymes operate within a narrow pH range; some have a
broad pH range.
12 Enzymes are also aff ected by the presence of inhibitors, which slow down their rate of reaction or
stop it completely.
13 Competitive inhibitors are molecules which are similar in shape to the normal substrate molecules.
Th ey compete for the active site of the enzyme. This type of inhibition is reversible because the inhibitor can enter and leave the active site.
14 Non-competitive inhibitors either bind permanently to the active site or bind at a site elsewhere on the enzyme, causing a change in shape of the active site. Such binding may or may not be reversible.
Multiple - choise Test
1 Which of the following describes an enzyme?
A a catalyst with an active site which binds to the product of a reaction
B a fibrous protein with an active site which binds to a substrate
C a globular protein with hydrophilic groups on its surface
D an insoluble biological catalyst
2 The graph shows the energy changes during the progress of a chemical reaction.

A 1, 2 and 3
B 1 and 2 only
C 1 and 3 only
D 2 and 3 only
3 Which tests, carried out on samples taken at intervals during the course of the reaction below, would enable the progress of the reaction to be followed?

1 Benedict?s test
2 biuret test
3 emulsion test
4 iodine in potassium iodide solution test
A 1 or 2
B 1 or 4
C 2 or 3
D 3 or 4
4 Which of the following describes the effects of temperature on an enzyme-controlled reaction?
A At low temperatures, substrate molecules only rarely collide with an enzyme?s active site.
B At low temperatures, the enzyme loses its shape and activity.
C At low temperatures, the enzyme becomes denatured.
D As the temperature increases, the number of collisions between enzyme and substrate decrease.
5 Which statement does not describe the effect on an enzyme?s activity of changing the pH.
A A pH that is very different from the enzyme?s optimum pH can denature the enzyme.
B At low pH there are fewer hydrogen ions to interact with the R groups of the amino acids that make up the enzyme.
C Changing the pH alters the interactions of the amino acids that make up the enzyme.
D Changing the pH alters the enzyme?s three-dimensional shape.
6 The graph shows the effect of increasing the substrate concentration on the rate of an enzyme-catalysed reaction.

What explains why the rate of reaction levels off?
A All the enzyme active sites are saturated with substrate.
B All the substrate has been used up.
C The concentration of the enzyme is gradually decreasing.
D The rate at which enzyme and substrate collide is decreasing.
7 Curve X shows the progress of an enzyme-catalysed reaction under optimum conditions.
Curve Y shows the same reaction with one factor changed throughout the reaction.

Which factor was changed?
A A competitive inhibitor was added.
B A non-competitive inhibitor was added.
C The reaction took place at a different temperature.
D The reaction took place at a different pH.
8 These statements are about enzyme inhibitors.
1 binds reversibly to an enzyme
2 binds to a site on the enzyme different from the active site
3 has a structural similarity to the enzyme?s normal substrate
4 can be displaced from the enzyme by a high concentration of the enzyme?s normal substrate
Which statements describe a competitive inhibitor?
A 1, 2, 3 and 4
B 1, 3 and 4 only
C 2 and 3 only
D 3 and 4 only
9 Fibrous protein from dead cells is difficult to remove from contact lenses. Some cleaning solutions contain an enzyme to digest this protein to soluble products.
What describes the enzyme and its activity?
A An active site on a fibrous protein binds to the enzyme and is hydrolysed.
B An active site on a soluble product binds to the enzyme and is digested.
C An active site on a globular protein binds to a soluble product and digests it.
D An active site on a globular protein binds to a fibrous protein and hydrolyses it.
10 Several different organisms produce lactase enzymes to hydrolyse lactose. The different enzymes have different molecular sizes.
Which description of these different lactases is correct?
A Their active sites have the same shape.
B Their primary structure is the same.
C They each contain the same number of amino acids.
D They have exactly the same three-dimensional structure.
Answer for Multiple - choise Test
1 C
2 C
3 B
4 A
5 B
6 A
7 B
8 B
9 D
10 A
End-of-chapter questions
1 The diagram below shows an enzyme and an inhibitor of the enzyme. Which of the following describes the inhibitor?
A competitive, irreversible B competitive, reversible
C non-competitive, irreversible
D non-competitive, reversible

2 In a reaction controlled by an enzyme, which of the following graphs shows the effect of substrate concentration on the rate of the reaction?

 3 The graph shows the progress of the digestion of starch by the enzyme salivary amylase. Why does the reaction slow down?
3 The graph shows the progress of the digestion of starch by the enzyme salivary amylase. Why does the reaction slow down?A End-product inhibition by maltose.
B The salivary amylase is becoming denatured.
C The salivary amylase is gradually becoming saturated with starch.
D There are fewer and fewer substrate molecules left to bind with the salivary amylase.
4 If methylene blue dye is added to a suspension of yeast cells, living cells do not take up the stain, and they remain colourless. However, dead cells are stained blue.This fact was used to carry out an investigation into the rate at which yeast cells were killed at two different temperatures (at high temperatures the yeast enzymes will be denatured).

Which of the following is correct?

5 Copy the graph in question 3 and draw a line from which the initial rate of reaction could be calculated.
6. The graph below shows the effect of changes in pH on the activity of the enzyme lysozyme.

a Describe the effect of pH on this enzyme.
b Explain why pH affects the activity of the enzyme.
7 The graph below shows the effect of temperature on the rate of reaction of an enzyme.

a What is indicated by X?
b What temperature would X be for a mammalian enzyme?
c Explain what is happening in region A.
d Explain what is happening in region B.
e Enzymes are effective because they lower the activation energy of the reactions they catalyse. Explain what is meant by 'activation energy'.
8 The reaction below occurs during aerobic respiration. The reaction is catalysed by the enzyme succinic dehydrogenase.

a Name the substrate in the above reaction. [1] b The molecule malonic acid, which is shown below, inhibits this reaction. It does not bind permanently to the enzyme. Describe how malonic acid inhibits the enzyme succinic dehydrogenase. [3]

i Name the amino acid which contains -SH groups.[1]
ii Explain the function of -SH groups in proteins and why binding of heavy metals to these groups would inhibit the activity of an enzyme.[4]
iii What type of inhibition would be caused by the heavy metals?[1]
[Total: 10]
9. You are provided with three solutions: A, B and C. One solution contains the enzyme amylase, one contains starch andone contains glucose. Starch is the substrate of the enzyme. The product is the sugar maltose. You are provided withonly one reagent, Benedict's solution, and the usual laboratory apparatus.
a Outline the procedure you would follow to identify the three solutions.[6]
b What type of reaction is catalysed by the enzyme? [1]
[Total: 7]
10 The activity of the enzyme amylase can be measured at a particular temperature by placing a sample into a Petri dish containing starch-agar ('a starch-agar plate'). Starch-agar is a jelly containing starch. One or more 'wells' (small holes) arecut in the agar jelly with a cork borer, and a sample of the enzyme is placed in each well. The enzyme molecules then diffuse through the agar and gradually digest any starch in their path. At the end of the experiment, iodine in potassium iodide solution is poured over the plate. Most of the plate will turn blue-black as iodine reacts with starch, but a clear 'halo' (circle) will be seen around the well where starch has been digested. Measuring the size of the halo cangive an indication of the activity of the enzyme.
A student decided to investigate the rate at which a mammalian amylase is denatured at 60°C. She heated different amples of the enzyme in a water bath at 60°C for 0, 1, 5, 10 and 30 minutes. She then allowed the samples to cool downto room temperature and placed samples of equal volume in the wells of five starch-agar plates, one plate for eachheating period. She then incubated the plates in an oven at 40°C for 24 hours.
The results of the student's experiment are shown on the next page. A diagram of one dish is shown, and the real size of one halo from each dish is also shown.
a Why did the student cut four wells in each dish rather than just one? [1]
b One dish contained samples from amylase which was not heated (time zero). This is a control dish. Explain the purpose of this control. [1]
c Explain why the starch-agar plates were incubated at 40°C and not room temperature. [1]
d Describe what was happening in the dishes during the 24 hours of incubation [4]
e Why was it important to add the same volume of amylase solution to each well? [1]
f Measure the diameter in mm of the representative halo from each dish. Record the results in a suitable table. [4]
g Only one halo from each dish is shown in the diagrams. In practice there was some variation in the diameters of the four halos in each dish. How would you allow for this when processing your data? [1]
h Plot a graph to show the effect of length of time at 60°C on the activity of the enzyme. [5]
i Describe and explain your results. [4]
j Another student discovered that amylases from fungi and bacteria are more resistant to high temperatures than mmalian amylases. Using starch-agar plates as a method for measuring the activity of an amylase at 40°C, outline an experiment that the student could perform to discover which amylase is most resistant to heat. Note that temperatures up to 120 °C can be obtained by using an autoclave (pressure cooker).
k Enzymes are used in many industrial processes where resistance to high temperatures is an advantage.[5]
State three other variables apart from temperature which should be controlled in an industrial process involving enzymes.[3]
[Total: 30]

Answer for end-of-chapter
1 B
2 D
3 D
4 C
5 straight line drawn from origin at zero to show steepest gradient of curve;
6 a maximum activity/optimum pH, is pH 5; activity gradually increases between pH 2 and
pH 5, and decreases from pH 5 to pH 10; activity very low at pH 2 and pH 10;
b pH is a measure of the hydrogen ion concentration; hydrogen ions are positively charged;
hydrogen ions can interact with the R groups of amino acids; affects ionic bonding/affects ionisation of R groups; affects tertiary structure/affects 3D shape of enzyme; therefore substrate may not fit active site (as precisely);
7 a optimum temperature;
b 37 °C accept 40 °C
c as temperature increases the kinetic energy of the molecules increases; the rate of collision between substrate and, enzyme/active site, increases; rate of reaction increases;
d the enzyme is gradually being denatured; when the rate is zero the enzyme is completely
denatured; enzyme loses tertiary structure; substrate no longer fits into active site/active site
loses its (specific) shape so substrate does not fit; hydrogen bonds broken/increased vibration of
enzyme molecule;
e the extra energy which must be given to the substrate; before it can be converted into the product;
Exam-style questions

8 a succinic acid; [1]
b malonic acid acts as a competitive inhibitor; it has a similar shape/structure to succinic acid;
it therefore competes with succinic acid for a place in the active site of the enzyme; [3]
c i cysteine; [1]
ii ?SH groups form disulfi de bridges; used to determine tertiary structure; heavy metal would prevent formation of disulfi de bridges; could change shape of active site; heavy metal could affect shape either by binding directly in the active site, or by binding at another site which then results in change in shape of the active site; substrate would not be able to fi t into active site; [max. 4]
iii (non-competitive) irreversible; [1]
[Total: 10]
8 a succinic acid; [1]b malonic acid acts as a competitive inhibitor;
it has a similar shape/structure to succinic acid;
it therefore competes with succinic acid for a place in the active site of the enzyme; [3]
c i cysteine; [1]
ii ?SH groups form disulfi de bridges;
used to determine tertiary structure;
heavy metal would prevent formation of disulfi de bridges;
could change shape of active site;
heavy metal could aff ect shape either by binding directly in the active site, or by binding at another site which then results in change in shape of the active site; substrate would not be able to fit into active site; [max. 4]
iii (non-competitive) irreversible; [1]
[Total: 10]
9 a carry out Benedict?s test on solutions A, B and C;a positive result/brick-red precipitate will be seen, with the glucose solution;
heat separate samples of the two remaining solutions, in boiling water bath/to high
temperature (e.g. 80 °C), for suitable time/at least two minutes (enzyme will be denatured);
for each heated solution, mix it with an unheated sample of the other solution;
leave several minutes/suitable time (for reaction to take place);
carry out Benedict?s test on the two tubes;
only one will give a positive result (due to presence of maltose) and this will be the one
which contained the unheated enzyme;
accept alternative wording for all steps in the procedure, provided the same logical sequence
is described [max. 6]
b hydrolysis; [1]
[Total: 7]
10 a replication increases reliability; AW [1]b to act as a reference to show what happens if there is no denaturation; AW [1]
c 40 °C is the optimum temperature for a mammalian enzyme; [1]
d enzyme/amylase (molecules) diff use(s) from wells into the agar;
enzyme/amylase digests the starch;
to maltose;
forms rings/halos, of digested starch around the wells;
amount of digestion/rate of digestion, is related to degree of denaturation of enzyme/amylase;[max. 4]
e the more enzyme/amylase added, the greater the amount of digestion of starch
or
want results to be due to diff erences in preheating times, not to diff erences in amount of amylase/enzyme; AW [1]
f

table drawn with ruled lines for border and to separate columns and headings (ideally ruled lines
between rows, but not essential for mark);
correct headings to columns with units;
first column is independent variable (Time heated at 60 °C);
correct measurements of halos; [4]
g measure the four halos and calculate the mean; [1]
(any anomalous results should be ignored)
h

units given on axes, min/minutes and mm;
regular intervals on both axes (check that 0, 1, 5, 10, 30 are not regularly spaced on x-axis);
points plotted accurately;
points joined with straight lines or smooth curve; [5]
i enzyme was completely denatured after 30 minutes;
rate of denaturation was rapid at first and then gradually slowed down;
data quoted;
enzyme loses tertiary structure;
substrate no longer fi ts into active site/active site loses its (specific) shape so substrate does not fit;
AVP e.g. hydrogen bonds broken/increased vibration of enzyme molecule; [max. 4]
j heat samples of mammalian, fungal and bacterial amylases at diff erent temperatures;
suitable range, e.g. between 40 °C and 120 °C;
40 °C is a control (for reference to fi nd out size of halo with no denaturation);
at least five temperatures, e.g. 40, 60, 80, 100, 120 °C;
heat for suitable length of time (e.g. one hour, at least ten min);
cool to room temp/40 °C, add equal volumes to wells in starch?agar plates, replicate wells in each
plate (e.g. four), leave 24 hours, test for starch, measure diameters of halos; [max. 5]
Background information: amylase enzymes from the bacterium Bacillus licheniformis and
the fungus Aspergillus have been developed by biotechnology companies for use in industrial
processes. For example, a bacterial amylase that functions in the range 90?110 °C has been
developed and is used in beer brewing and other processes, and a fungal amylase that operates in
the range 50?60 °C is used for pastry baking and maltose syrup production.
k pH ;
substrate concentration;
enzyme concentration; [3]
[Total: 30]
- Q: Explain The Effects Of Too High A Temperature On An Enzyme
If the temperature is too high, an increase in kinetic energy of the molecules will cause excessive vibrations of the molecules. This will disrupt the 3 dimensional structure of the enzyme, causing it to become a random coil. The enzyme is therefore denatured....
- Q: Distinguish Between Competitive And Non-competitive Inhibition Of Enzymes
A competitive inhibitor is structurally similar to the substrate but a non-competitive inhibitor is often structurally different from the substrate. Hence, a competitive inhibitor will compete with the substrate for an active site but a non competitive...
- #20.factors Affecting The Rate Of Enzyme-catalysed Reactions
These factors are: - Temperature - pH - Enzyme concentration - Substrate concentration - Inhibitor concentration When an enzyme solution is added to a solution of its substrate, the molecules collide. With time, the quantity of substrate...
- #17.2 Enzymes - Syllabus 2016
3.1 Mode of action of enzymes 3.2 Factors that affect enzyme action Enzymes are essential for life to exist. Their mode of action and the factors that affect their activity are explored in this section. Prior...
- #17.1 Enzymes - Syllabus 2015
? Mode of action of enzymes ? Factors that affect enzyme action Learning Outcomes Candidates should be able to: (a) explain that enzymes are globular proteins that catalyse metabolic reactions; (b) explain the mode of action of enzymes in terms of...
